Schlüsselbegriffe: regionale Effekte des Klimawandels, Lymantria dispar, Euproctis chrysorrhoea, Temperatur, UV-Strahlung
Available at https://doi.org/10.53203/fs.2301.1
See below the issue 1/2023 as E-Paper or have a look at our E-Paper archive dating back to 1955.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Abstract
The impact of climate change on insect pests is an emerging topic in forestry and forest science. This study investigates the relationships between two broadleaved forest pests – spongy moth (Lymantria dispar L.) and brown-tail moth (Euproctis chrysorrhoea L.) – and oaks (Quercus sp.) as their hosts. Oak forests cover almost one-third of the total forest area of Serbia and are ecologicallyvery valuable, but at the same time vulnerable, as being affected in adverse ways by several primary pests and pathogens. Since 1862, Serbia experienced several extremely large outbreaks of spongy moth with more than a hundred thousand hectares completely defoliated each time, while brown-tail moth occurred periodically with a much lower spatial extent. The aim of this research was to investigate the effect of UV radiation (UVR) and air temperature on spongy moth and brown-tail moth in Serbian forests. We used simulations of the coupled regional climate model EBU-POM (Eta Belgrade University-Princeton Ocean Model) for the A1B scenario for the period 2001-2030 as main input and different statistical methods to explore relationships between observations of pest spread and climate change impacts. Our results suggest
(i) increasing the areas affected by spongy moth due to its sensitivity on UVR in May, and
(ii) altitudinal spreading of brown-tail moth population up to 800 – 1000 m.
This research indicates that in situ forest observations in Serbia are not only affected by climate change, but also by the combined effect of climate on forest pests. For further research, we recommend exploring other forest stressors or dieback phenomena in European forests by applying the same or similar regional climate model dataset.
Zusammenfassung
Die Auswirkungen des Klimawandels auf Schadinsekten sind ein aktuelles Thema für die Forstwirtschaft und Forstwissenschaften. In diesem Beitrag wird die Beziehung zwischen zwei Laubholzschädlingen – dem Schwammspinner (Lymantria dispar L.) und dem Goldafter (Euproctis chrysorrhoea L.) – und den Eichen (Quercus sp.) als Wirtspflanzen untersucht. Eichenwälder bedecken fast ein Drittel der gesamten Waldfläche Serbiens und sind ökologisch sehr wertvoll, aber gleichzeitig auch anfällig, da sie stark von mehreren Primärenschädlingen und Krankheitserregern betroffen sind. Seit 1862 kam es in Serbien zu mehreren extrem großen Ausbrüchen durch den Schwammspinner, bei denen jedes Mal mehr als hunderttausend Hektar Wald vollständig kahlgefreßen wurden, während der Goldafter periodisch aber in viel geringerem Ausmaß auftritt. Ziel dieser Studie war es, die Auswirkungen von UV-Strahlung (UVR) und Lufttemperatur auf Schwammspinner und Goldafter in serbischen Wäldern zu untersuchen. Wir haben Simulationen des gekoppelten regionalen Klimamodells EBU-POM (Eta Belgrade University-Princeton Ocean Model) für das A1B-Szenario für den Zeitraum 2001–2030 und verschiedene statistische Methoden verwendet, um die Beziehung zwischen Beobachtungen der Ausbreitung von Schadinsekten und dem Ausmaß der Auswirkungen des Klimawandels zu untersuchen. Unsere Ergebnisse zeigen
(i) Vergrößerung der vom Schwammspinner befallenen Flächen aufgrund seiner Empfindlichkeit gegenüber UV-Strahlung im Mai und
(ii) eine Höhenausbreitung der Goldafterpopulation bis zu 800–1000 m Seehöhe.
Wir konnten zeigen, dass vor Ort erhobene Waldmessungen in Serbien nicht nur stark vom Klimawandel, sondern auch durch Schadinsekten beeinflusst werden. Für zukünftige Forschungen empfehlen wir, weitere Stressfaktoren und andere Absterbephänomene in europäischen Wäldern mittels desselben oder ähnlicher Klimamodelldatensätze zu untersuchen.
1 Introduction

Figure 1: Images (a) spongy moth larvae; (b) brown-tail moth larvae; (c) complete defoliation of Hungarin oak forest caused by spongy moth in Negotin county during outbreak in 2013; (d) Sessile oak dieback after two subsequent defoliation by brown-tail moth in the western part of Serbia.
Abbildung 1: Bilder von (a) Schwammspinnerlarven; (b) Goldafterlarven; (c) vollständiger Kahlfraß des ungarischen Eichenwaldes durch den Schwammspinner im Bezirk Negotin während des Ausbruchs im Jahr 2013; (d) Absterbeerscheinungen der Traubeneiche nach zwei aufeinanderfolgenden Kahlfraßereignissen durch den Goldafter im Westen Serbiens.
Forest ecosystems are threatened by many factors with both, biotic and abiotic origins (Machado Nunes Romeiro et al. 2022). Oak forests are ecologically very valuable, but also very vulnerable covering almost one-third of the total forested area of Serbia (Banković et al. 2009). They are affected vastly by several primary pests such as spongy moths (Lymantria dispar L.) and brown-tail moths (Euproctis chrysorrhoea L.) (Fig. 1). Knowledge about the factors that are driving spongy moth outbreaks is very valuable for both, current and future pest management, especially due to of expected climate changes, which may alter the interaction between oaks as hosts and their pests. The impact of climate change on insect pests is an emerging topic in forestry science (Pureswaran et al. 2018, Jactel et al. 2019). Climate change is referred to as “the biggest global health threat of the 21st century” (Costello et al. 2009). We expand that statement by adding the term “plant health”. This term was used in Döring et al. (2012), where the authors highlighted that health risk sources are clearly defined, while others, usually occurring indirectly and in interaction with factors affected by climate change, are not so well-defined.
The analysis outputs from several general circulation models suggest that the countries in Southeastern Europe, including Serbia, are facing significant impacts of climate change, affecting all aspects of human life. Several studies describe climate change issues for Serbia or its regions through dynamic and statistical downscaling and examination of the effect of climate change on crop yield, viticulture, climate indices, and partly forests (Mihailović et al. 2015). However, none of the studies was devoted to the issue of the impact of climate change, such as air temperature and UV radiation (UVR), on forest pest insects causing widespread damage to both ecology and human health in Serbia. Climate change is altering various components of the Earth's climate system affecting both, the amount of ozone in the stratosphere, and UV radiation exposure. Changes in UV levels can be a direct consequence of changes in ozone, or they may be indirectly affected by changes in other factors such as clouds, UV-absorbing tropospheric gases, aerosols, or surface reflectance (Bais et al. 2015, McKenzie et al. 2011).
Tree defoliators, such as are spongy moth (Lymantria dispar L.), as the most harmful forest pests in the northern hemisphere (Montgomery & Wallner 1988), and the brown-tail moth (Euproctis chrysorrhoea L.), have a huge economic impact on forest ecosystems. According to Janković (1954), the spongy moth can be found in all parts of Serbia, even up to 1600 m.a.s.l., but only below 1000 m it is considered to be a significant pest. From 1862 to 1995, Serbia experienced 16 spongy moth outbreaks (Marović et al. 1998), and another three up to 2014 (Mihajlović et al. 1998, Mihajlović 2008, Milanović 2014a). Some of them had disastrous consequence such as outbreak culmination in 1957 when spongy moths attacked 50% of all forests in Serbia (Milević 1959), while outbreaks in 1997 and between 2004 and 2008 were less severe, with about 25% of all Serbian forests being attacked (Mihajlović et al. 1998, Mihajlović 2008). The last outbreak began in 2011 and it was lasting until 2014. The spongy moth completely defoliated 66,000 ha of oak and beech forests in Serbia in 2013, while 65,000 ha suffered from severe defoliation during the outbreak in the same year (Milanović et al. 2014b). Frequent outbreaks of spongy moths, with two or more subsequent defoliation, results in a delayed fruting (Gottschalk 1990), great loss of radial growth in current and later years (Muzika & Liebhold 1999, 2001, Naidoo & Lechowicz 2001, Fajvan et al. 2008), and ending with a forest decline in the following years (Davidson et al. 1999). These pests may also threaten human as well as animal health during outbreaks (Kikuchi et al. 2012) due to the strong allergenic impact of toxins released from broken caterpillars’ setae. On one hand, climate changes affect forest pests either in a straight line or through the changes of host plants or natural enemies (Anttila et al. 2010), which is usually seen through the impact of temperature, precipitation, and UVR. On the other hand, spongy moth may affect ecosystem processes directly or indirectly (Gandhi & Herms 2010) through the chain reactions (Kenis et al. 2009), and the quality and amount of water available downstream (Lovett et al. 2002, Kretchun et al. 2014). Forests respond to elevated UV radiation indirectly with an increased level of leaf phenolics and flavonoids (Sullivan 2005). These plant secondary metabolites that enhance UV protection effectiveness may also alter leaf development, water relations, and plant-herbivore interactions (Rousseaux et al. 1998, 2004).
For the assessment of the climate change impact on two oak defoliators in Serbia for the period 2001-2030, we used the outputs of the EBU-POM regional climate model with the A1B scenario over the period 2001-2030 and the reference climate simulations for the period 1961-1990. We put the focus on the following points:
(i) effect of UVR on spongy moth population dynamic in the most affected part of Serbia using the projected values of the monthly UVR doses and
(ii) movement of altitude of brown-tail moth outbreaks in dependence on a vertical shift of Köppen climate zones in Serbia.
2 Materials and methods
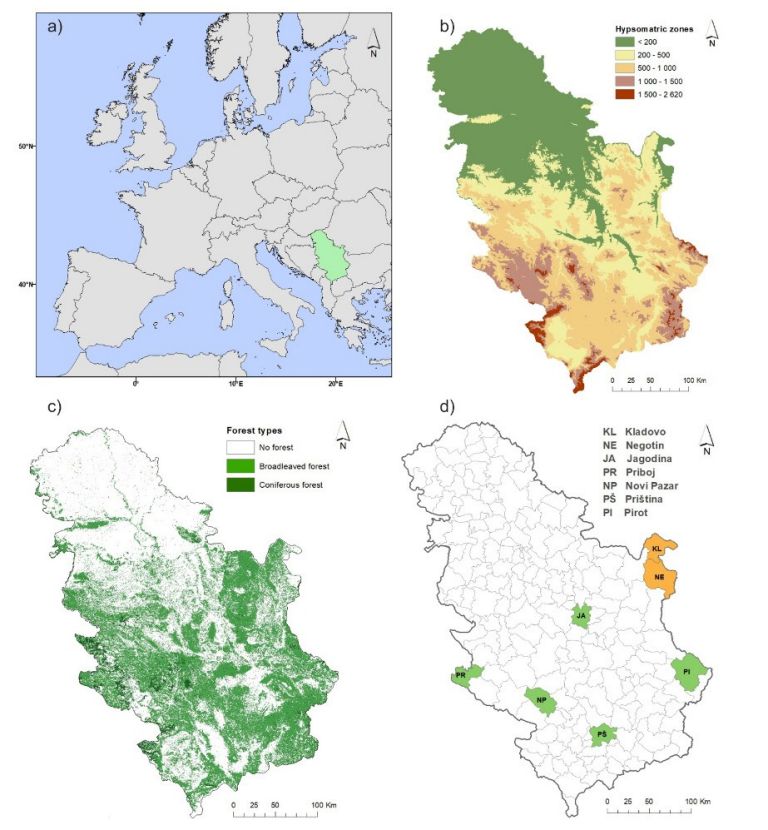
Figure 2: (a) Location of Serbia in Europe; (b) elevation zones; (c) forest cover; (d) NUTS 4 (Nomenclature of Territorial Units for Statistics) units included in study (brown-tail moth [green]; spongy moth [brown]).
Abbildung 2: (a) Lage Serbiens in Europa; (b) Höhenzonen; (c) Waldbedeckung; (d) NUTS 4 (Systematik der Gebietseinheiten für die Statistik) Einheiten, die in die Studie einbezogen wurden (Goldafter [grün]; Schwammspinner [braun]).
2.1 Study area and climate
The study area is Serbia, located between latitudes 41° and 47° N and longitudes 18° and 23° E. It covers a total of 88,361 km2 (Figs. 2a and 2b). A map of the distribution of forest covers in Serbia used in this study is given in Fig. 2c while studying areas for forest insects are depicted in Fig. 2d. According to the Köppen classification, the climate zones in Serbia include Cfwax”, Cfwbx”, Dfwbx” and “ET”. Additional information about climate details for Serbia is availble in Mihailović et al. (2015).
2.2 Models and formula used
2.2.1 The global and regional climate models
Dynamical downscaling of the results of the global climate model ECHAM5 coupled with the Max Planck Institute Ocean Model was used to obtain time series of essential climate variables over Serbia for the period 1951-2100. For the downscaling, coupled regional climate model EBU-POM was employed. The atmospheric part of this model is the Eta/NCEP model (EBU-Eta Belgrade University) and the ocean part is the Princeton Ocean Model (POM). The atmospheric part of the model covered most of the European continent, while the ocean part was defined over the Mediterranean Sea. The coupling frequency between the two components was six minutes. The horizontal resolutions of the atmospheric and ocean model were 25 km and 20 km, respectively. The number of vertical levels in the atmospheric model was 32 and in the ocean model 21. Coupling was performed following the request of energy and mass conservation on the interface between air and water, without any flux correction add-ons. From the atmospheric model, radiation, turbulent, and mass fluxes were used as a forcing input for the ocean model, and on the other hand, sea surface temperature from the ocean model was used as a bottom boundary condition for the atmospheric model (Đurđević & Rajković 2012). Over the open seas outside the Mediterranean basin, the bottom boundary condition was defined using the results of the global climate model.
2.2.2 Empirical formulae
For calculating the daily doses of UVR radiation, i.e. UVRd, for study area sites we have used the empirical formula derived by Malinović-Milićević et al. (2013), i.e.
where Gd is the daily sum of the global solar radiation (kJ/m2) depending on altitude.
2.3.2 Forest pest insects
Observed records on spongy moth and brown-tail moth that include data on the annually infested areas were collected for the period 1991-2020 in the central part of Serbia. A combined transect and method of temporary sample plots (Vasić 1981) were applied to detect the presence of spongy moth egg masses and brown-tail moth larval nests in the forest during autumn each year. In brief, all forest compartment were cheked during the Autumn each year, by the staff of the Forest State Enterprise “Srbijšume”, when all trees and spongy moth egg masses were counted on the transect line. Based on the collected data about the checked trees and found spongy moth egg masses, from the field observation, and the number of trees per area unit, obtained from the forest managements plans, population density for spongy moth were determined and expressed as the number of egg masses per hectar. Population density of spongy moth was classified as outbreaking if the more than 10 egg masses per hectare were recorded in specific forest compartment. To increase the accuracy of the population density assesmenent, at least four temporary sample plots 10 by 10 meteres in size, were established in each compartment where an increase in population density was recorded. For the brown-tail moth, due to high mortality of the overwintering larvae, there is no reliable threshold for the damage assesmnent or expected population density. Therefore, additional visual assessment of possible forest defoliation was done during the next spring for both species. All collected data were supervised and processed by the experts in forest protection from the Institute of forestry in Belgrade, from the central part of Serbia, authorized by the Forest Directorate (Ministry of Agriculture, Forestry and Watermanagement). Since 2013, all annual reports related to forest protection are publicly available on the Institute of forestry website (https://www.forest.org.rs).
Based on the available data, maps of spongy moth distribution in Serbia during the last three outbreaks were constructed. After these maps were overlapped with each other and polygons representing each outbreak intersected, the areas (polygons) with a different frequency of spongy moth occurrence during these three outbreaks were formed (Figure 3a).
2.4 Statistics
The calculations were performed for the entire time interval 1961-1990 using data taken from daily meteorological reports of the Republic Hydrometeorological Service of Serbia. To establish a vertical shift of the brown-tail moth population in outbreaks and its dependence on the mean annual temperature, regression analysis was applied. Linear regression analysis was established to statistically analyze the impact of UVR on the areas annually affected by a spongy moth (SMAA) with different time-lag periods. To test the effect of the SMAA during the previous years autocorrelation (ACF) and partial autocorrelation (PCF) analysis was applied for the summed data for the Negotin and Kladovo counties in OriginPro software ver. 2023 (OriginLab Corporation, Northampton, MA, USA).
3 Results
3.2 Forest insects
Over the period 1991-2020, there were three spongy moth outbreaks in Serbia with devastating proportions. We have observed that eastern Serbia was most affected by spongy moth, especially the area of Negotin and Kladovo counties (Fig. 2d) where spongy moth occurs during each outbreak (Fig. 3a). We considered the time-lag effect response of spongy moth culmination to the UVR stronger influence, during April, May, and June, when larval feeding is most intensive.

Figure 3: (a) Spatial distribution of the three spongy moth outbreaks during the period 1995-2014. The blue ellipse indicates the location of Negotin county and Kladovo county (Fig. 2d), (b) annual areas affected by SMAA versus the sum of the UVR doses for May shifted for six years and (c) temporal distribution of annual areas affected by a spongy moth (SMAA) in thousands of hectares, and the UVR doses in May.
Abbildung 3: (a) Räumliche Verteilung der drei Ausbrüche des Schwammspinners im Zeitraum 1995–2014. Die blaue Ellipse zeigt die Lage der Bezirke Negotin und Kladovo (Abb. 2d), (b) jährliche vom SMAA betroffene Flächen versus der Summe der um sechs Jahre verschobenen UVR-Dosis für Mai und (c) Zeitreihe der jährlichen von dem Schwammspinner (SMAA) betroffenen Flächen in Tausend Hektar und die UVR-Dosis im Mai.

Figure 4: Significance of the relationship between spongy moth outbreak areas at culmination and UVR doses in April, May, and July and with different time-lag.
Abbildung 4: Signifikanz der Beziehung zwischen Ausbruchsgröße vom Schwammspinner und UVR-Dosis im April, Mai und Juli mit unterschiedlicher zeitlicher Verzögerung.
We have calculated daily UVR doses under Eq. (1) using global radiation outputs from the EBU-POM model for the period April-September. These calculations were performed for selected Negotin county in Serbia. The relative change of the UVR doses in Serbia has increased with a tendency to be three times higher in the northern and northeastern (where the NE and KL sites are located) regions (1.2%) than in the central part of the country (0.4%). The EBU-POM model shows a significant increase of 14% in the number of days with maximum temperature over 30 °C (hot days), compared to the reference period 1961-1990, as well as a decrease of 17% in the number of days with a maximum temperature higher than 25 °C (warm days). This results in prolonged exposure of forest area to the UVR.

Figure 5: Time-series statistics for yearly affected area by spongy moth from Negotin and Kladovo counties (1991–2020) shown in Fig. 3c. (a) Autocorrelation function, (b) Partial autocorrelation function.
Abbildung 5: Zeitreihenstatistiken für das jährlich von Schwammspinner betroffene Gebiet aus den Bezirken Negotin und Kladovo (1991–2020), dargestellt in Abb. 3c. (a) Autokorrelationsfunktion, (b) partielle Autokorrelationsfunktion.
To establish the effect of monthly UVR doses (obtained from the EBU-POM model) on SMAA we considered their dependence on the time scale for different time lags in April, May, and June. This was done by changing the time lag for one year, stepping backward, and starting from the outbreak year. For each time lag regression analysis was applied (SMAA against monthly UVR doses). It is found that only UVR doses in May have a significant effect on SMAA (p < 0.01) during outbreak culmination with a time lag of six years (Fig. 4). This strong relationship is visualized in Fig. 3b where the coefficient of correlation (r = 0.657, p < 0.05) points that out. Fig. 3c shows the temporal distribution of SMAA and the UVR doses in May shifted for six years. Looking at this figure we can see that significant variation of SMAA during spongy moth outbreaks could be addressed to the monthly UVR doses in May. Additional analisys reveal that at the 0.05 level the autocorrelation function is not significantly different from zero (χ2 = 10.40, p = 0.238), which implay that SMAA was not driven by the affected area during the previous years. Lack of significance in ACF and PCF for different time lags is depicted in the Fig. 5.

Figure 6: Effect of the shift in altitude of the Köppen climate zones of Serbia on the vertical spread of brown-tail moth population outbreaks observed for five sites in Serbia (the shifts in altitude were derived from the EBU-POM model simulations under the A1B scenario for the period 2001-2030).
Abbildung 6: Auswirkung der Höhenverschiebung der Köppen-Klimazonen Serbiens auf die vertikale Ausbreitung von Ausbrüchen der Goldafterpopulation, die für fünf Standorte in Serbien beobachtet wurden (die Höhenverschiebungen wurden aus den EBU-POM-Modellsimulationen unter dem A1B-Szenario für den Zeitraum 2001–2030 abgeleitet).
Before 1990 brown-tail moth outbreaks in Serbia were usually recorded at altitudes up to 600 m). However, in the last three decades (1991-2020), this pest has been found in counties at altitudes higher than 600 m, i.e. up to 1,000 m (Fig. 2d). This coincides with the fact that in the first third of the 21st century (period 2001-2030) the Köppen climate zones of Serbia will move in height. For instance, Cfvbk” type from 600-800 m to 1,000 m and higher, compared to the simulations of EBU-POM model (A1B scenario) for the period 1961-1990. The mountain climate (Dfvbk”) will move by about 100 m and an increase in the mean annual temperature. Fig. 6 shows that the spreading of the brown-tail moth population with altitude (h) can be attributed to the increase in the mean annual temperature in those regions of Serbia since h and air temperature are positively correlated (r = 0.671; p < 0.05).
4 Discussion
It is expected that much more damage to Serbian forests would come from spongy moth and brown-tail moth, which are pests typical for deciduous forests that mostly cover the territory of Serbia (Fig. 2c). Their importance overcome even Europe and Asia since both species were introduced in North America during the 19th century (Liebhold et al. 1992, Schaeffer, 1974). According to Davidson et al. (1999) after 2-3 years of spongy moth consecutive heavy defoliation, oak tree mortality rate on dry sites can reach 80% in America, while in Europe mortality rate is estimated up to 30% (McManus & Csoka 2007). There are few papers dealing with the quantification of the relationship between forest insects and climate change and long-term impacts in forests damage. Klapwijk et al. (2013) considered five forest pests including the brown-till moth exhibiting outbreak dynamics in Hungary. They performed analysis using general linear models and generalized least squares regression related to mean monthly temperature and precipitation in conditions when temperature increased considerably, especially over the last 25 years (1.6 °C) while the amount of precipitation exhibited no trend over this period. Additionally, an increase of the defoliated forest by brown-tail moth in Main (USA) was favoured by the higher temperature during August and September in the previous year (Boyd et al. 2021). Thus, defoliated area in the same study increased from 1,000 hectares in 1995 to 50,000 hectares in 2018. We established a relationship between shifts in altitude of the Köppen climate zones of Serbia projected by the regional climate model and observed changes in altitudinal distribution of brown-tail moth, threatening larger areas of deciduous forests in Serbia (Fig. 6).
Spongy moth affects a large area of Serbian forests with very frequent outbreaks (Milanović et al. 2014b) with a high probability to be continued in the future due to climate change impact (Hlásny & Turčáni 2008). Thus, the climate warming scenarios predict shift of the spongy moth distribution northern boundary by 500–700 km, while the southern edge will retract northwards by 100-900 km (Vanhanen et al. 2007). More recent study of Fält-Nardmann et al. (2018) limited the shift of the spongy moth northern boundary to 200-300 kilometers mainly due to extremely low temperatures during winter. Population size, and consequently area affected by forest pests, is regulated by endogenous and exogenus factors (Royama 1992). Endogenous factors, such as natural enemies, are density dependent while exogenous factors, such as weather condition, act independently of population density (Williams & Liebhold 1995a). However, delayed effect of both regulators on the population size can be detected by autocorrelation and partial correlation functions (Williams & Liebhold 1995b, Liebhold et al. 2000). Lack of significance in ACF and PCF for different time lags can be explained by the suppression measures against spongy moth which probabily interapt the natural processes. Therefore, we focused on the exogenous factors to explain temporal variation of SMAA in the eastern part of Serbia. Phytophagous insects respond to solar UVR directly by avoiding exposure and indirectly by plant-mediated changes in host tissue (Mazza et al. 1998). Exposure to UVR increases the content of plant secondary metabolites, including phenolic compounds in leaves (Gourlay et al. 2022), that may affect insect herbivores (Izaguirre et al. 2007) by binding proteins in the insect’s midgut (Schultz 1989). Analyzing results obtained by several authors (Anttila et al. 2010, Buck & Callaghan 1999, Ballaré et al. 1996, Netherer & Schopf 2010, Rajput et al. 2022) and our results, we assumed that spongy moth population density was affected indirectly by UVR through its effect on leaf quality. As it is reported by many authors, an increase in leaf secondary metabolites and a decrease in nutrients lead to reduced food consumption and growth of the spongy moth (Barbehenn et al. 2013, Milanović et al. 2016, Solla et al. 2016). To our knowledge, there are no papers dealing with the intensity of the influence of UVR on spongy moth outbreaks.
In the years with decreased UVR doses in May, when most intensive feeding of the spongy moth larvae occurs, leaves have a reduced level of secondary metabolites that allow spongy moth larvae to grow better and produce females with increased fertility compared to the years with increased UVR. It could be reasonable to hypothesize that the reduced amount of UVR doses in May will steadily increase SMAA which was confirmed for the three outbreaks in Negotin and Kladovo counties during the period 1991-2020. Presumably, the reduced amount of UVR could be a trigger for the outbreak to come after a certain period of years depending on ecological conditions. In our study, the period was six years as it is shown in Fig. 4.
A lot of hypotheses that explain the population dynamics of forest pests (see Myers 1993) have in common mechanisms that delay the recovery of the population following a decline of insect outbreaks. For example, the nutrient stress hypothesis (Tuomi 1984) that explains delays of 3-4 years in leaf quality recovery after insect defoliation can be used to explain the missing culmination of the spongy moth outbreak in 2016.
5 Conclusion
In this paper, we analyzed the climate change impact (A1B scenario for the period 2001-2030) on two forest pests in Serbia affecting its environment. This impact is estimated based on in situ observations of forest insects and their alternations for the time period 1991-2020. We have drawn the following conclusions:
(i) the effect of increasing the UV radiation on the spongy moth population dynamic, in the most affected part of Serbia, shows a decrease in its population, and
(ii) there exists an altitudinal spreading of brown-tail moth population because, according to Köppen classification, the Dfwbx” zone (mountain climate) retreats giving space to warmer Cfwbx” zone (moderate continental).
List of abbreviations
Cfwax … continental climate
Cfwbx … moderate continental
Dfwbx … mountain climate
ET … Polar climate group; Tundra precipitation type
ECHAM5 … The fifth-generation atmospheric general circulation model ECHAM5 developed at the Max-Planck Institute for Meteorology
Eta/NCEP … National Center for Environmental Prediction
EBU-POM … Eta Belgrade University- Princeton Ocean Model
POM … Princeton Ocean Model
SMAA … Areas Annually Affected by Spongy Moth.
RP01-30 … Reference Period for the 2001-2030 in climate simulations
SRES-A1B … Special Report on Emissions Scenarios for A1B
UVR … UV Radiation
Acknowledgments
SDM and DTM conceived the study and helped to draft the manuscript. SDM and ZT carried out the field data collection, VDJ, SMM, and ML generated data from the models about the UVR and Temperature for the research area. SM, SDM and DTM performed the statistical analysis, ZT visualize the results and performed. All authors contributed equally to the writing of the final manuscript version. This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia, grant numbers 451-03-47/2023-01/200169, 451-03-68/2022-14/200172, 451-03-68/2022-14/200117, and 451-03-47/2023-01/200015.
References
Anttila U., Julkunen-Tiitto R., Rousi M., Yang S., Rantala J.M., Ruuhola T. 2010. Effects of elevated ultraviolet-B radiation on a plant-herbivore interaction. Oecologia, 164, 163-175. doi.org/10.1007/s00442-010-1658-5
Bais A.F., McKenzie R.L., Bernhard G., Aucamp P.J., Ilyas M., Madronich S., Tourpali K. 2015. Ozone depletion and climate change: impacts on UV radiation, Photochemical & Photobiological Sciences, 14, 19-52. https://doi.org/10.1039/c4pp90032d
Ballaré, C.L., Scopel, A.L., Stapleton, A.E., Yanovsky, M.J. 1996. Solar Ultraviolet-B Radiation Affects Seedling Emergence, DNA Integrity, Plant Morphology, Growth Rate, and Attractiveness to Herbivore Insects in Datura ferox. Plant Physiology, 112(1), 161-170. https://doi.org/10.1104/PP.112.1.161
Banković S., Medarević M., Pantić D., Petrović N., Šljukić B., Obradović S. 2009. The growing stock of the Republic of Serbia – State and problems. Bulletin Faculty of Forestry, 100, 7-29, (In Serbian with English summary) doi.org/10.2298/GSF0900007B
Barbehenn R.V., Niewiadomski J., Pecci C., Salminen J.P. 2013. Physiological benefits of feeding in the spring by Lymantria dispar caterpillars on red oak and sugar maple leaves: nutrition versus oxidative stress. Chemoecology, 23, 59-70, doi.org/10.1007/s00049-012-0119-5
Boyd K. S., Drummond F., Donahue C., Groden E. 2021. Factors Influencing the Population Fluctuations of Euproctis chrysorrhoea (Lepidoptera: Erebidae) in Maine, Environmental Entomology, 50, 5, 1203–1216, https://doi.org/10.1093/ee/nvab060
Buck N., Callaghan T. V. 1999. The direct and indirect effects of enhanced UV-B on the moth caterpillar Epirrita autumnata. Ecological Bulletins, 47, 68-76.
Costello A., Abbas M., Allen A., Ball S., Bell S., Bellamy R., Friel S., Groce N., Johnson A., Kett M., Lee M., Levy C., Maslin M., McCoy D., McGuire B., Montgomery H., Napier D., Pagel C., Patel J., de Oliveira J.A.P., Redclift N., Rees H., Rogger D., Scott J., Stephenson J., Twigg J., Wolff J., Patterson C. 2009. Managing the health effects of climate change. The Lancet 373(9676): 1693-1733. doi.org/10.1016/S0140-6736(09)60935-1
Davidson C.B., Gottschalk K.W., Johnson J.E. 1999. Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: a review. Forest Sci. 45: 74-84
Döring T.F., Pautasso M., Finckh M.R., Wolfe M.S. 2012. Concepts of plant health – reviewing and challenging the foundations of plant protection. Plant Pathology, 61, 1-15. doi.org/10.1111/j.1365-3059.2011.02501.x
Đurđević V., Rajković B. 2012. Development of the EBU-POM coupled regional climate model and results from climate change experiments in: Mihailović D.T., Lalić B. (Eds.) Advances in environmental modeling and measurements. Nova Science Publishers Inc, New York, pp 23-32.
Fajvan, M.A., Rentch, J., Gottschalk, K. 2008. The effects of thinning and gypsy moth defoliation on wood volume growth in oaks. Trees 22, 257–268. https://doi.org/10.1007/s00468-007-0183-6
Fält-Nardmann J.J.J., Ruohomäki K., Tikkanen O.-P. Neuvonen S. 2018, Cold hardiness of Lymantria monacha and L. dispar (Lepidoptera: Erebidae) eggs to extreme winter temperatures: implications for predicting climate change impacts. Ecological Entomolology, 43, 422-430. https://doi.org/10.1111/een.12515
Gandhi K.J.K., Herms D.A. 2010. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biological Invasions,12, 389-405. doi.org/10.1007/s10530-009-9627-9
Gottschalk K.W. 1990. Gypsy moth effects on mast production. In McGee C.E. (ed.): Proceedings of the Workshop: Southern Appalachian Mast Management. University of Tennessee, Knoxville, pp. 42-50
Gourlay G., Hawkins B.J., Albert A., Schnitzler J.P., Constabel P.C. 2022. Condensed tannins as antioxidants that protect poplar against oxidative stress from drought and UV-B. Plant, Cell and Environment, 45, 362–377, doi.org/10.1111/pce.14242
Hlásny T., Turčáni M. 2008. Insect pests as climate change driven disturbances in forest ecosystems in: Strelcova K., Matyas C., Kleidon A., Lapin M., Matejka F., Blazenec M., Škvarenina J., Holecy J. (Eds.) Bioclimatology and Natural Hazards. Springer, Berlin, pp 165-178. doi.org/10.1007/978-1-4020-8876-6_15
Izaguirre M.M., Mazza C.A., Svatos A., Baldwin I.T., Ballaré C.L. 2007. Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Annals of Botany, 99, 1, 103-109, doi.org/10.1093/aob/mcl226
Jactel H., Koricheva J., Castagneyrol B. 2019. Responses of forest insect pests to climate change: not so simple, Current Opinion in Insect Science 35: 103-108, doi.org/10.1016/j.cois.2019.07.010
Janković, L.J. (1954). Some observations on the height distribution of gypsy moth. Plant Protection. 23, 103-104.
Kenis M., Auger-Rozenberg M.A., Roques A., Timms L., Péré C., Cock M.J.W., Settele J., Augustin S., Lopez-Vaamonde C. 2009. Ecological effects of invasive alien insects. Biological Invasions 11,1, 21-45. doi.org/10.1007/s10530-008-9318-y
Kikuchi T., Kobayashi K., Sakata K., Akasaka T. 2012. Gypsy moth-induced dermatitis: a hospital review and community survey. European Journal of Dermatology, 22, 384-390.
Klapwijk M.J., Csóka G., Hirka A., Björkman C. 2013. Forest insects and climate change: long-term trends in herbivore damage. Ecology and Evolution, 3, 4183-4196. doi.org/10.1002/ece3.717
Kretchun A.M., Scheller R.M., Lucash M.S., Clark K.L, Hom J., Van Tuyl S. 2014. Predicted Effects of Gypsy Moth Defoliation and Climate Change on Forest Carbon Dynamics in the New Jersey Pine Barrens. Plos One 9 e102531, 1-11. doi.org/10.1371/journal.pone.0102531
Liebhold A.M., Halverson J. A., Elmes G.A. 1992. Gypsy Moth Invasion in North America: A Quantitative Analysis. Journal of Biogeography, 19, 5, 513-520. https://doi.org/10.2307/2845770
Liebhold A., Elkinton J., Williams D., Muzika R.M. 2000. What causes outbreaks of the gypsy moth in North America? Population Ecology. 42, 257-266.
Lovett G.M., Christenson L.M., Groffman P.M., Jones C.G., Hart J.E., Mitchell M.J. 2002. Insect Defoliation and Nitrogen Cycling in Forests: Laboratory plot and watershed studies indicate that most of the nitrogen released from forest foliage as a result of defoliation by insects is redistributed within the ecosystem whereas only a small fraction of nitrogen is lost by leaching. BioScience, 52, 335-341. doi.org/10.1641/0006-3568(2002)052[0335:IDANCI]2.0.CO;2
Machado Nunes Romeiro J., Eid T., Antón-Fernández C., Kangas A., Trømborg E. 2022. Natural disturbances risks in European Boreal and Temperate forests and their links to climate change – A review of modelling approaches. Forest Ecology and Management, 509, 120071. doi.org/10.1016/J.FORECO.2022.120071
Malinović-Milicević S., Mihailović D.T., Lalić B., Drešković N. 2013. Thermal environment and UV-B radiation indices in the Vojvodina region Serbia. Climate Research 57, 111-121. doi.org/10.3354/cr01163
Marović, R., Maravić, M., Jančić, G., Lazarev V. 1998. Gypsy moth outbreaks in Serbia, In: Gypsy Moth Outbreaks in Serbia. (Ed. Ž. Adamović), 1-12. The Entomological Soci-ety of Serbia, Belgrade.
Mazza C.A., Zavala J., Scopel A.L., Ballaré C.L. (1999). Perception of solar UVB radiation by phytophagous insects: behavioral responses and ecosystem implications. Proceedings of the National Academy of Sciences, USA. 96, 3, 980-5. doi: 10.1073/pnas.96.3.980.
McKenzie R.L., Aucamp P.J., Bais A.F., Björn L.O., Ilyas M., Madronich S. (2011) Ozone depletion and climate change: impacts on UV radiation, Photochemical & Photobiological Sciences, 10, 182-198. https://doi.org/10.1039/c0pp90034f
McManus M., Csoka G. 2007. History and impact of gypsy moth in North America and comparison of recent outbreaks in Europe. Acta Silvatica et Lignaria Hungarica, 3, 47-64.
Mihailović D.T., Lalić B., Drešković N., Mimić G., Đurđević V., Jančić M. 2015. Climate change effects on crop yields in Serbia and related shifts of Köppen climate zones under the SRES-A1B and SRES-A2. International Journal of Climatology, 35, 3320-3334. doi.org/10.1002/joc.4209
Mihajlović, L.J. 2008. The gypsy moth (Lymantria dispar L.) (Lepidoptera: Lymantriidae) in Serbia. Forestry 1-2, 1-29.
Mihajlović, L.J., Grbić, P., Vandić D.1998. The latest outbreaks of gypsy moth, Lymantria dispar L., in the region of Serbia in the period 1995-1998, In: Gypsy Moth Outbreaks in Serbia. (Eds. Ž. Adamović), 81-88. The Entomological Society of Serbia, Belgrade.
Milanović S., Janković-Tomanić M., Kostić I., Kostić M., Morina F., Živanović B., Lazarević J. 2016. Behavioural and physiological plasticity of gypsy moth larvae to host plant switching. Entomologia Experimentalis et Applicata 158: 152-162. doi.org/10.1111/eea.12388
Milanović S., Mihajlović L, Marković N. 2014b. Gypsy moth in Serbia: status and prospects Entomology (2014b)., Grand Challenges Beyond Our Horizons, 15-19 November, Portland, USA, https://esa.confex.com/esa/2014/webprogram/Paper85668.html
Milanović S., Mihajlović L., Karadzić D., Jankovsky L., Aleksic P., Janković-Tomanić M., Lazarević J. 2014a. Effects of pedunculate oak tree vitality on gypsy moth preference and performance. Archives of Biological Sciences, 66, 1659-1672. doi.org/10.2298/ABS1404659M
Milević K. 1959. A review of the suppression of gypsy moth in P.R Serbia in the gradation from 1952 to 1957, Plant Protection 52-53, 121-144
Montgomery M.E., Wallner W. 1988. The gypsy moth a westward migrant in: Berryman A.A. (Ed.) Dynamics of forest insect populations patterns causes implications. Plenum Press, New York, 354-378. doi.org/10.1007/978-1-4899-0789-9_18
Muzika R.M., Liebhold A.M. 1999. Changes in radial increment of host and nonhost tree species with gypsy moth defoliation. Canadian Journal of Forest Research. 29, 9, 1365-1373. https://doi.org/10.1139/x99-098
Myers J. 1998. Synchrony in Outbreaks of Forest Lepidoptera: A Possible Example of the Moran Effect. Ecology, 79, 3, 1111-1117. doi.org/10.2307/176606
Naidoo R., Lechowicz M..J. 2001: The effects of gypsy moth on the radial growth of deciduous trees. Forest Science. 47, 338-348.
Netherer, S., Schopf, A. (2010). Potential effects of climate change on insect herbivores in European forests – General aspects and the pine processionary moth as specific example. Forest Ecology and Management, 259(4), 831-838. https://doi.org/10.1016/J.FORECO.2009.07.034
Pureswaran D.S., Roques A., Battisti A. 2018. Forest Insects and Climate Change, Current Forestry Reports, 4, 35-50, doi.org/10.1007/s40725-018-0075-6
Rajput, S., Puranik, N., Verma, S.K. (2022). Interaction of UV-B with Terrestrial Ecosystem. 341–352. https://doi.org/10.1007/978-981-19-3620-3_16
Rousseaux M.C., Julkunen-Tiitto R., Searles P.S. et al. (2004.) Solar UV-B radiation affects leaf quality and insect herbivory in the southern beech tree Nothofagus antarctica, Oecologia, 138, 505–512. doi.org/10.1007/s00442-003-1471-5
Rousseaux, M., Ballaré, C., Scopel, A. et al. 1998. Solar ultraviolet-B radiation affects plant-insect interactions in a natural ecosystem of Tierra del Fuego (southern Argentina). Oecologia 116, 528-535. doi.org/10.1007/s004420050618
Royama T. 1992. Analytical population dynamics. Chapman & Hall, London.
Schaefer P.W. 1974. The population ecology of the browntail moth (Euproctis chrysorrhoea) (Lepidoptera: Lymantriidae) in North America. Dissertation University of Maine, Orono, ME.
Schultz J.C. 1989. Tannin-Insect Interactions. In: Hemingway, R.W., Karchesy, J.J., Branham, S.J. (eds) Chemistry and Significance of Condensed Tannins. Springer, Boston, MA. doi.org/10.1007/978-1-4684-7511-1_26
Solla A., Milanović S., Gallardo A., Bueno A., Corcobado T., Cáceres Y., Morcuende D., Quesada A., Moreno G., Pulido F. 2016. Genetic determination of tannins and herbivore resistance in Quercus ilex. Tree Genetics and Genomes 12(117). doi.org/10.1007/s11295-016-1069-9
Sullivan J.H. 2005. Possible impacts of changes in UV-B radiation on North American trees and forests, Environmental Pollution, 137, 3, 380-389, doi.org/10.1016/j.envpol.2005.01.029
Tuomi J., Niemelä P., Haukioja E., Sirén S., Neuvonen S. 1984. Nutrient stress: an explanation for plant anti-herbivore responses to defoliation. Oecologia, 61, 208-210.
Vanhanen H., Veteli T.O., Paivinen S., Kellomaki S., Niemela P. 2007. Climate change and range shifts in two insect defoliators: gypsy moth and nun moth-a model study. Silva Fennica, 41(4), 621.
Vasić K. (ed.) 1981. Manual for diagnostical prognosis service of forest protection. Association of engineers and technicians of forestry and wood technology of Yugoslavia Belgrade.
Williams D.W., Liebhold A.M. 1995a. Influence of weather on the synchrony of gypsy moth (Lepidoptera: Lymantriidae) outbreaks in New England. Environmental Entomology, 24, 987–995.
Williams D.W., Liebhold A.M. 1995b Detection of delayed density dependence: effects of autocorrelation in an exogenous factor. Ecology, 76, 1005-1008.